ORYZON reports financial results and corporate updates for half-year ending June 30, 2023
- Positive aggregate safety data from vafidemstat's PORTICO Phase IIb trial in Borderline Personality Disorder (BPD), consistent with safety data from seven completed clinical trials, supporting the drug is safe and well-tolerated
- Continues to enroll patients in Phase IIb EVOLUTION trial with vafidemstat in schizophrenia
- Continues to recruit patients in FRIDA trial with iadademstat in combination with gilteritinib in relapsed/refractory FLT3-mutant AML patients
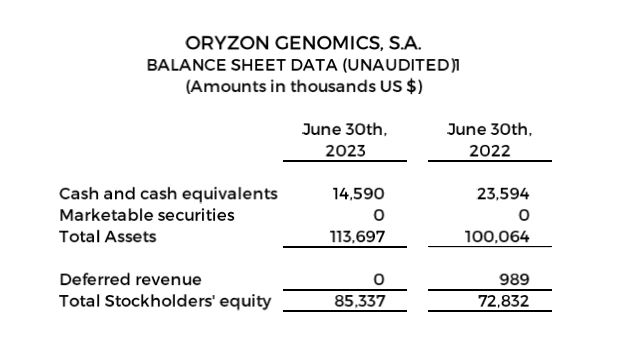
- Cash, cash equivalents and marketable securities of $14.6 million as of June 30, 2023
MADRID, SPAIN and BOSTON, MA, UNITED STATES, July 24th, 2023 – Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company leveraging epigenetics to develop therapies in diseases with a strong unmet medical need, today reported financial results for the half-year ended June 30, 2023 and provided a corporate update on recent developments.
Dr Carlos Buesa, Oryzon’s Chief Executive Officer, said: “Oryzon continued with a strong path in its clinical programs in 2Q23. In CNS, after obtaining positive results from the predefined interim analysis in the Phase IIb PORTICO trial with vafidemstat in BPD, we reported positive blinded aggregate safety data from 167 patients. BPD is a highly unmet medical need and an enormous commercial opportunity with limited competition and a low risk of generification. Recruitment of patients in PORTICO in the U.S. and Europe has proceeded at a very high rate, and we expect to analyze top-line data at the end of this year or early next year. Our Phase IIb trial with vafidemstat in schizophrenia, EVOLUTION, has also continued to enroll patients. We remain on track to submit the IND to initiate HOPE this year, the first randomized Phase I/II personalized medicine trial with an LSD1 inhibitor, in Kabuki Syndrome patients.”
Dr Buesa continued: “In oncology, we also continued to make progress this quarter, with both iadademstat’s ongoing clinical trials, the FRIDA trial in combination with gilteritinib in relapsed/refractory FLT3-mutant AML patients and the collaborative trial with the Fox Chase Cancer Center (FCCC) in combination with paclitaxel in neuroendocrine tumors, actively recruiting patients. In addition, and broadening our epigenetic pipeline, we reported positive preclinical efficacy data of ORY-4001, our selective HDAC-6 inhibitor recently nominated as a clinical development candidate, in Charcot-Marie-Tooth (CMT) disease. CMT is a progressive, degenerative disease involving the peripheral nerves, affecting 150,000 Americans and more than 3 million people around the world. It is one of the most prevalent conditions among rare diseases and currently lacks effective treatments or cures.”
Second Quarter and Recent Highlights
Vafidemstat in large multifactorial CNS indications:
- Positive preliminary blinded aggregate safety data from PORTICO, vafidemstat’s Phase IIb clinical trial in BPD, reported following the recent independent Data Monitoring Committee (DMC) meeting on June 26th, corresponding to the initial 167 randomized patients (data cut-off, May 23rd 2023). There were no treatment-related serious adverse events or deaths. An aggregated number of 306 adverse events, affecting 98 patients treated either with vafidemstat or placebo were reported, most of them were mild (216) or moderate (78), with only 12 reported as severe, in 9 patients, leading to 6 treatment discontinuations or patient withdrawals. The reviewed blinded PORTICO safety data is aligned with aggregated safety data collected from seven completed vafidemstat clinical trials, in which almost 400 subjects have been treated with the drug. The independent DMC provided the recommendation to continue the trial without modifications until full enrollment, which is planned for early Q3 2023. Current data of PORTICO continue to support that vafidemstat is safe and well-tolerated. Positive results from PORTICO’s interim analysis, conducted by the independent DMC in Q1 2023, were previously reported, with the trial being determined to be non-futile and to be continued with the planned enrollment number. PORTICO is a multicenter, double-blind, randomized, placebo-controlled Phase IIb conducted in the US and EU to evaluate the efficacy and safety of vafidemstat in BPD patients. The trial has two independent primary objectives: reduction of aggression/agitation and overall BPD improvement. The study aims to include about 188 patients, distributed between two arms.
- The EVOLUTION Phase IIb clinical trial with vafidemstat in patients with schizophrenia has continued to enroll patients. This Phase IIb study aims to evaluate the efficacy of vafidemstat on negative symptoms and cognitive impairment in patients with schizophrenia. This project is partially financed with public funds from the Spanish Ministry of Science and Innovation and is being carried out in various Spanish hospitals.
Vafidemstat in monogenic CNS indications:
- We are finalizing the preparation of a new precision medicine trial in Kabuki Syndrome (KS). This Phase I/II trial, named HOPE, will be a multicenter, multi-arm, randomized, double-blind and placebo-controlled trial to explore the safety and efficacy of vafidemstat in improving several impairments described in KS patients. The trial plans to enroll 50-60 patients and will be carried out in several hospitals and sites in the United States and, possibly, in Europe. The company is in a dialogue with the regulatory agencies to refine the final design of this trial and expects to submit the IND for HOPE to the FDA in 2023.
- Our precision medicine programs in psychiatric disease continue to progress. We have collaborations in autism with researchers at the Seaver Autism Center for Research and Treatment at the Icahn School of Medicine at Mount Sinai Hospital in New York and the Institute of Medical and Molecular Genetics (INGEMM) at Hospital Universitario La Paz of Madrid and in schizophrenia with researchers from Columbia University in New York. The analysis of the results of the pilot studies to characterize patients with specific mutations to inform subsequent precision psychiatry clinical trials with vafidemstat is ongoing.
Iadademstat in oncology:
- FRIDA, an open-label, multicenter Phase Ib clinical trial of iadademstat in combination with gilteritinib in patients with relapsed/refractory (R/R) Acute Myeloid Leukemia (AML) harboring a FMS-like tyrosine kinase mutation (FLT3mut+), has continued to enroll patients. The primary objectives of the trial are to evaluate the safety and tolerability of iadademstat in combination with gilteritinib in patients with FLT3mut+ R/R AML and to establish the Recommended Phase 2 Dose (RP2D) for this combination. Secondary objectives include the evaluation of the treatment efficacy, measured as the rate of complete remission and complete remission with partial hematological recovery (CR/CRh), the Duration of Responses (DoR), and the assessment of Measurable Residual Disease. The study is being conducted in the USA and will accrue up to approximately 45 patients. If successful, Oryzon and the FDA have agreed to hold a meeting to discuss the best plan to further develop this combination in this much-in-need AML population.
- The collaborative Phase II basket trial of iadademstat in combination with paclitaxel in platinum R/R small cell lung cancer (SCLC) and extrapulmonary high-grade neuroendocrine tumors (NET trial) has continued to enroll patients. This trial is conducted in the US under a collaborative clinical research agreement with the Fox Chase Cancer Center (FCCC), under which the FCCC will be conducting different collaborative combination clinical trials with iadademstat, with Oryzon providing funding, the drug, and technical expertise.
- Preparations for new trials in combination in solid tumors are continuing. In SCLC, the STELLAR trial, a randomized, multicenter Phase Ib/II study of iadademstat plus a checkpoint inhibitor in first-line extensive-stage SCLC, is being prepared. The company believes that STELLAR could potentially support an application for accelerated approval.
Earlier stage programs:
- Positive preclinical efficacy data in CMT with ORY-4001, a selective histone deacetylase 6 (HDAC-6) inhibitor, was presented at the 2023 Peripheral Nerve Society annual meeting (PNS-2023) held in June. ORY-4001 treatment was able to reverse disease progression symptoms in a CMT mice model which reliably recapitulates many of the symptoms of this condition in humans. Notably, ORY-4001 was able to improve myelination and restore axon integrity in the sciatic nerve, and improved compound muscle action potential and nerve conduction in comparison with untreated animals. The results presented are fruit of a collaboration entered in 2022 between Oryzon and the CMT Research Foundation (CMTRF), a U.S.-based patient-led, non-profit organization focused on delivering treatments and cures for CMT. ORY-4001 was recently nominated as a clinical development candidate for the treatment of certain neurological diseases as CMT, Amyotrophic Lateral Sclerosis (ALS) and others, and the compound will now enter into IND enabling studies to prepare it for clinical studies. HDAC6 inhibitors have been previously proposed as potentially effective treatments for CMT, ALS and other neurological disorders that lack effective treatments.
Financial Update: First Half 2023 Financial Results
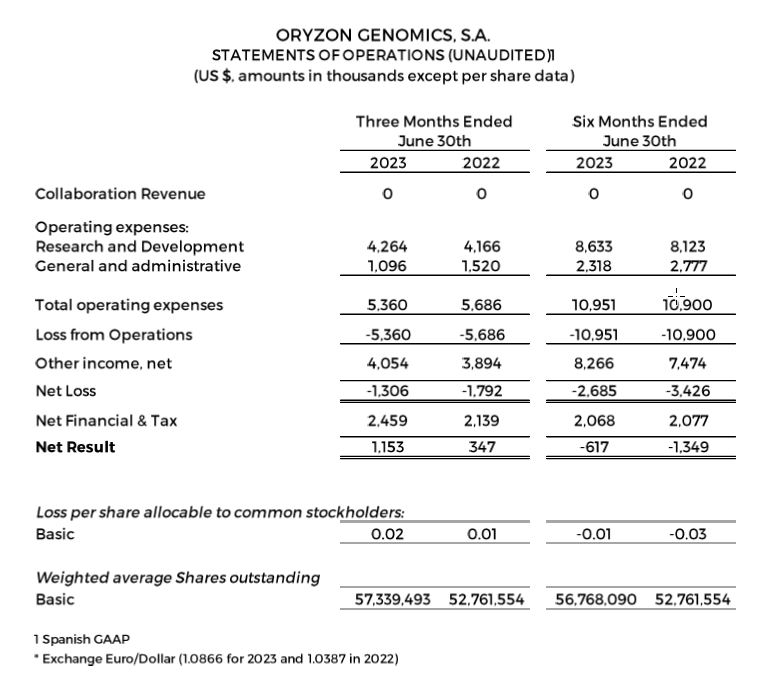
Research and development (R&D) expenses were $4.3 and $8.6 million for the quarter and six months ended June 30, 2023, compared to $4.2 and $8.1 million for the quarter and six months ended June 30, 2022.
General and administrative expenses were $1.1 and $2.3 million for the quarter and six months ended June 30, 2023, compared to $1.5 and $2.8 million for the quarter and six months ended June 30, 2022.
Net losses were $1.3 and $2.7 million for the quarter and six months ended June 30, 2023, compared to $1.8 and $3.4 million for the quarter and six months ended June 30, 2022. The result is as expected, given the biotechnology business model where companies in the development phase typically have a long-term maturation period for products, and do not have recurrent income.
Negative net result was $0.6 million (-$0.01 per share) for the six months ended June 30, 2023, compared to a negative net result of $1.3 million (-$0.03 per share) for the six months ended June 30, 2022
Cash, cash equivalents and marketable securities totaled $14.6 million as of June 30, 2023.
About Oryzon
Founded in 2000 in Barcelona, Spain, Oryzon (ISIN Code: ES0167733015) is a clinical stage biopharmaceutical company and the European leader in epigenetics, with a strong focus on personalized medicine in CNS disorders and oncology. Oryzon’s team is composed of highly qualified professionals from the pharma industry located in Barcelona, Boston, NYC and San Diego. Oryzon has an advanced clinical portfolio with two LSD1 inhibitors, vafidemstat in CNS and iadademstat in oncology, in several Phase II clinical trials. The company has other pipeline assets directed against other epigenetic targets. In addition, Oryzon has a strong platform for biomarker identification and target validation for a variety of malignant and neurological diseases. For more information, visit
About Iadademstat
Iadademstat (ORY-1001) is a small oral molecule, which acts as a highly selective inhibitor of the epigenetic enzyme LSD1 and has a powerful differentiating effect in hematologic cancers (see Maes et al., Cancer Cell 2018 Mar 12; 33 (3): 495-511.e12.doi: 10.1016 / j.ccell.2018.02.002.). A FiM Phase I/IIa clinical trial with iadademstat in R/R AML patients demonstrated the safety and good tolerability of the drug and preliminary signs of antileukemic activity, including a CRi (see Salamero et al, J Clin Oncol, 2020, 38(36): 4260-4273. doi: 10.1200/JCO.19.03250). In a recently completed Phase IIa trial in elder 1L-AML patients (ALICE trial), iadademstat has shown encouraging safety and efficacy data in combination with azacitidine (see Salamero et al., ASH 2022 oral presentation). Iadademstatis currently being evaluated in combination with gilteritinib in the Phase Ib FRIDA trial in patients with relapsed/refractory AML with FLT3 mutations. Beyond hematological cancers, the inhibition of LSD1 has been proposed as a valid therapeutic approach in some solid tumors such as small cell lung cancer (SCLC), neuroendocrine tumors (NET), medulloblastoma and others. In a Phase IIa trial in combination with platinum/etoposide in second line ED-SCLC patients (CLEPSIDRA trial), preliminary activity and safety results have been reported (see Navarro et al., ESMO 2018 poster). Iadademstat is being evaluated in a collaborative Phase II basket study with the Fox Chase Cancer Center in combination with paclitaxel in R/R neuroendocrine carcinomas, and the company is preparing a new trial in combination in SCLC. Oryzon has entered into a Cooperative Research and Development Agreement (CRADA) with the U.S. National Cancer Institute (NCI) to collaborate on potential further clinical development of iadademstat in different types of solid and hematological cancers. In total iadademstat has been dosed so far to more than 100 cancer patients in four clinical trials. Iadademstat has orphan drug designation for SCLC in the US and for AML in the US and EU.
About Vafidemstat
Vafidemstat (ORY-2001) is an oral, CNS-optimized LSD1 inhibitor. The molecule acts on several levels: it reduces cognitive impairment, including memory loss and neuroinflammation, and at the same time has neuroprotective effects. In animal studies vafidemstat not only restores memory but reduces the exacerbated aggressiveness of SAMP8 mice, a model for accelerated aging and Alzheimer’s disease (AD), to normal levels and also reduces social avoidance and enhances sociability in murine models. In addition, vafidemstat exhibits fast, strong, and durable efficacy in several preclinical models of multiple sclerosis (MS). Oryzon has performed two Phase IIa clinical trials in aggressiveness in patients with different psychiatric disorders (REIMAGINE) and in aggressive/agitated patients with moderate or severe AD (REIMAGINE-AD), with positive clinical results reported in both. Additional finalized Phase IIa clinical trials with vafidemstat include the ETHERAL trial in patients with Mild to Moderate AD, where a significant reduction of the inflammatory biomarker YKL40 has been observed after 6 and 12 months of treatment, and the pilot, small-scale SATEEN trial in Relapse-Remitting and Secondary Progressive MS, where anti-inflammatory activity has also been observed. Vafidemstat has also been tested in a Phase II in severe Covid-19 patients (ESCAPE) assessing the capability of the drug to prevent ARDS, one of the most severe complications of the viral infection, where it showed significant anti-inflammatory effects in severe Covid-19 patients. Currently, vafidemstat is in two Phase IIb trials in borderline personality disorder (PORTICO) and in schizophrenia patients (EVOLUTION). The company is also deploying a CNS precision medicine approach with vafidemstat in genetically-defined patient subpopulations of certain CNS disorders and is preparing a clinical trial in Kabuki Syndrome patients. The company is also exploring the clinical development of vafidemstat in other neurodevelopmental syndromes.
FORWARD-LOOKING STATEMENTS
This communication contains, or may contain, forward-looking information and statements about Oryzon, including financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, capital expenditures, synergies, products and services, and statements regarding future performance. Forward-looking statements are statements that are not historical facts and are generally identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although Oryzon believes that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Oryzon shares are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Oryzon that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the documents sent by Oryzon to the Spanish Comisión Nacional del Mercado de Valores (CNMV), which are accessible to the public. Forward-looking statements are not guarantees of future performance and have not been reviewed by the auditors of Oryzon. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date they were made. All subsequent oral or written forward-looking statements attributable to Oryzon or any of its members, directors, officers, employees or any persons acting on its behalf are expressly qualified in their entirety by the cautionary statement above. All forward-looking statements included herein are based on information available to Oryzon on the date hereof. Except as required by applicable law, Oryzon does not undertake any obligation to publicly update or revise any forward‐looking statements, whether as a result of new information, future events or otherwise. This press release is not an offer of securities for sale in the United States or any other jurisdiction. Oryzon’s securities may not be offered or sold in the United States absent registration or an exemption from registration. Any public offering of Oryzon’s securities to be made in the United States will be made by means of a prospectus that may be obtained from Oryzon or the selling security holder, as applicable, that will contain detailed information about Oryzon and management, as well as financial statements.