ORYZON reports financial results and corporate update for quarter ended December 31, 2023
- Topline data from vafidemstat's PORTICO Phase IIb trial in Borderline Personality Disorder (BPD) reported with promising results in secondary endpoints of overall severity and control of agitation/aggression
- Company planning to request an End-of-Phase II meeting with the FDA to discuss plans for a registrational Phase III trial in BPD
- Continues to enroll patients in Phase IIb EVOLUTION trial with vafidemstat in schizophrenia
- Continues to recruit patients in FRIDA trial with iadademstat in combination with gilteritinib in relapsed/refractory FLT3-mutant AML patients
- Research and development (R&D) expenses of $3.9 and $16.6 million for the quarter and twelve months ended December 31, 2023, respectively
MADRID, SPAIN and BOSTON, MA, UNITED STATES, February 26th, 2024 – Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company leveraging epigenetics to develop therapies in diseases with a strong unmet medical need, today reported financial results for the fourth quarter ended December 31, 2023 and provided a corporate update on recent developments.
Dr Carlos Buesa, Oryzon’s Chief Executive Officer, said: “Oryzon continued with a strong path in its clinical programs in the fourth quarter. In CNS, we recently presented topline results for our Phase IIb PORTICO trial evaluating vafidemstat as a treatment for Borderline Personality Disorder (BPD). Although the primary endpoints were not met, two important secondary endpoints evaluating improvement in overall BPD disease severity and in agitation/aggression reached nominal statistical significance, and the results across all the primary and secondary efficacy endpoints favored vafidemstat over placebo. This is the first time, to the best of our knowledge, that a large, randomized Phase II trial in BPD had two secondary endpoints that met statistical significance reflecting clinically meaningful improvements in overall BPD severity and in agitation/aggression. In a disease with currently no approved drugs, and no established regulatory endpoints yet, PORTICO’s results across these two important endpoints are paving the way, in our opinion, to define a registrational Phase III trial, based for the first time on a well-controlled study that has enrolled a truly representative real-world BPD population. Once the full analysis is completed, the company will request an End-of-Phase II meeting with the FDA to discuss the design of a Phase III. Our Phase IIb trial with vafidemstat in schizophrenia, EVOLUTION, has also continued to enroll patients. We continue working for a future submission of an IND to initiate HOPE in 2024, the first randomized Phase I/II personalized medicine trial with an LSD1 inhibitor, in Kabuki Syndrome patients.”
Dr Buesa continued: “In oncology, we also continued to make progress this quarter in both iadademstat’s ongoing clinical trials. In the FRIDA trial in combination with gilteritinib in relapsed/refractory FLT3-mutant AML patients, the second cohort is fully enrolled and ongoing, and the company’s assessment on the initial preliminary data looks very promising when compared to gilteritinib alone in historical data. We expect to communicate some preliminary data at the EHA Conference next June in Madrid. The collaborative trial with the Fox Chase Cancer Center (FCCC) in combination with paclitaxel in neuroendocrine tumors is also actively recruiting patients. In addition, we are expanding iadademstat’s clinical development through additional clinical trials under our CRADA with the NCI and through investigator-initiated studies in SCLC in combination with ICIs and in first line AML in combination with venetoclax and azacitidine.”
Dr Buesa added: “While we have experienced a clear advance in our clinical pipeline, on the financial side, the company has continued its budgetary discipline. This, and the combination of non-dilutive funds (private and public grants and loans from commercial Spanish banks) with the withdrawal of €10 million from our €45 million Convertible Bond program allows us to extend the runway until 2025 and to focus now on the next conversations with the FDA and EMA and our clinical execution.”
Fourth Quarter and Recent Highlights
Vafidemstat in large multifactorial CNS indications:
- Topline results from PORTICO, a multicenter, double-blind, randomized, placebo-controlled Phase IIb conducted in the U.S. and EU to evaluate the efficacy and safety of vafidemstat in BPD patients, were reported on January 5, 2024. The primary endpoints, improvement in Borderline Personality Disorder Checklist (BPDCL) and in agitation/aggression by the Clinical Global Impression – Severity Agitation/Aggression (CGI-S A/A), did not reach statistical significance. However, nominal statistical significance was achieved on the secondary endpoint Borderline Evaluation of Severity (BEST), an overall measure of BPD disease severity, at weeks 8-12 (p = 0.042), with a relative reduction observed in the vafidemstat-treated group over the placebo group of 28.9%. Nominal statistical significance was also achieved on the secondary endpoint State-Trait Anger Expression Inventory 2 (STAXI-2) Trait Anger, a measure of agitation and aggression, at weeks 8-12 (p = 0.026), with a relative reduction observed in the vafidemstat-treated group over the placebo group of 46.7%. Results across all primary and secondary efficacy endpoints consistently favored vafidemstat over placebo. Global Statistical Test (GST p-values) confirmed a consistent trend across efficacy endpoints. Vafidemstat was safe and well-tolerated. Adverse events (AEs) were generally consistent with the safety profile of vafidemstat seen to date, with no new safety findings. Based on the efficacy and safety results, Oryzon intends to request an end-of-Phase II meeting with the FDA to discuss plans for a registrational Phase III study for the treatment of BPD. The company is currently completing the full data analysis and plans to provide a full data presentation at a psychiatric conference later this year, as well as in a peer-reviewed journal publication.
- The EVOLUTION Phase IIb clinical trial with vafidemstat in patients with schizophrenia continues to enroll patients. This Phase IIb study aims to evaluate the efficacy of vafidemstat on negative symptoms and cognitive impairment in patients with schizophrenia. This project is partially financed with public funds from the Spanish Ministry of Science and Innovation and is being carried out in various Spanish hospitals.
Vafidemstat in monogenic CNS indications:
- We continue the preparations of a new precision medicine trial in Kabuki Syndrome (KS). The company is in a dialogue with the regulatory agencies to refine the final design of this trial and expects to submit an IND for HOPE to the U.S. Food and Drug Administration (FDA) in 2024.
Iadademstat in oncology:
- FRIDA, an open-label, multicenter Phase Ib clinical trial of iadademstat in combination with gilteritinib in patients with relapsed/refractory (R/R) Acute Myeloid Leukemia (AML) harboring a FMS-like tyrosine kinase mutation (FLT3mut+), continues to enroll patients. The first cohort has been completed (six patients), and the combination was safe and showed strong antileukemic activity. The second cohort (six patients) is fully enrolled and ongoing. The primary objectives of the trial are to evaluate the safety and tolerability of iadademstat in combination with gilteritinib in patients with FLT3mut+ R/R AML and to establish the Recommended Phase 2 Dose (RP2D) for this combination. Secondary objectives include the evaluation of the treatment efficacy, measured as the rate of complete remission and complete remission with partial hematological recovery (CR/CRh), the Duration of Responses (DoR), and the assessment of Measurable Residual Disease (MRD). The study is being conducted in the U.S. and will accrue up to approximately 45 patients. If successful, Oryzon and the FDA have agreed to hold a meeting to discuss the best plan to further develop this combination in this much-in-need AML population.
- The Company is further expanding the clinical development of iadademstat in AML through an Investigator-initiated study (IIS). This trial will be a Phase Ib dose-finding study to evaluate iadademstat in combination with venetoclax and azacitidine in first-line AML patients, led by Oregon Health & Science University (OHSU). The trial received FDA IND approval in 4Q2023 and is expected to begin enrolling patients in 1Q2024.
- The collaborative Phase II basket trial of iadademstat in combination with paclitaxel in platinum R/R small cell lung cancer (SCLC) and extrapulmonary high-grade neuroendocrine tumors (NET trial) continues to enroll patients. This trial is being conducted in the U.S. under a collaborative clinical research agreement with the FCCC, under which the FCCC will be conducting different collaborative combination clinical trials with iadademstat, with Oryzon providing funding, the drug, and technical expertise.
- A new clinical trial of iadademstat in combination with an immune checkpoint inhibitor (ICI) in first-line metastatic SCLC, which will be conducted under the Cooperative Research and Development Agreement (CRADA) signed with the National Cancer Institute (NCI) in the United States, is under preparation. This trial will be led by the Memorial Sloan Kettering Cancer Center (MSKCC), which plans to file the IND with the FDA in 1Q2024.
- The STELLAR trial, a randomized, multicenter Phase Ib/II study of iadademstat plus a checkpoint inhibitor in first-line extensive-stage SCLC, will be informed and refined from the findings of the CRADA-MSKCC trial in the same space and with the same design that is expected to start in 1Q2024, as mentioned above. The company believes that STELLAR could potentially support an application for accelerated approval.
Earlier stage programs:
- ORY-4001, Oryzon’s highly selective histone deacetylase 6 (HDAC6) inhibitor nominated as a clinical candidate for the treatment of certain neurological diseases such as Charcot-Marie-Tooth disease (CMT), Amyotrophic Lateral Sclerosis (ALS) and others, is progressing through IND enabling studies to prepare it for clinical studies. The Company recently announced that is has received a 0.5 million USD grant from the ALS Association in the U.S. to support the regulatory preclinical development of ORY-4001 for ALS. ORY-4001 has been previously shown to reverse disease progression symptoms in a CMT mice model which reliably recapitulates many of the symptoms of this condition in humans, improving myelination and restoring axon integrity in the sciatic nerve, and improving compound muscle action potential and nerve conduction. These results are fruit of a collaboration entered in 2022 between Oryzon and the CMT Research Foundation (CMTRF), a U.S.-based patient-led, non-profit organization focused on delivering treatments and cures for CMT.
- Oryzon has received two new grants to further explore the role of epigenetic targets in the treatment of neuronal pathologies. These are two collaborative projects with public research centers, focused on the discovery and validation of novel biomarkers and epigenetic targets for the treatment of neuronal pathologies. The projects have a global budget of 2.3 million euros, of which Oryzon will receive up to 1.4 million euros.
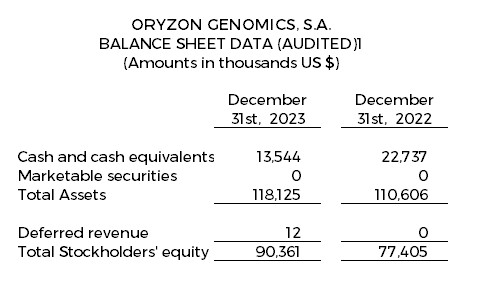
Financial Update: Fourth Quarter 2023 Financial Results
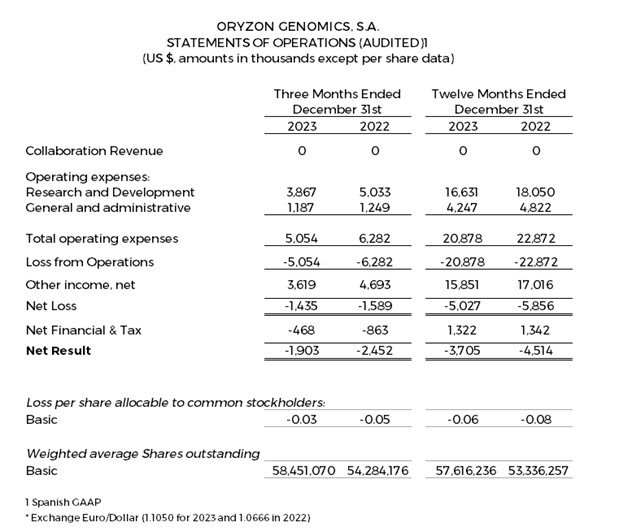
Research and development (R&D) expenses were $3.9 and $16.6 million for the quarter and twelve months ended December 31, 2023, respectively, compared to $5.0 and $18.1 million for the quarter and twelve months ended December 31, 2022.
General and administrative expenses were $1.2 and $4.2 million for the quarter and twelve months ended December 31, 2023, respectively, compared to $1.2 and $4.8 million for the quarter and twelve months ended December 31, 2022.
Net losses were $1.4 and $5.0 million for the quarter and twelve months ended December 31, 2023, respectively, compared to $1.6 and $5.9 million for the quarter and twelve months ended December 31, 2022. The result is as expected, given the biotechnology business model where companies in the development phase typically have a long-term maturation period for products, and do not have recurrent income.
Negative net result was $3.7 million (–$0.064 per share) for the twelve months ended December 31, 2023, compared to a negative net result of $4.5 million (–$0.085 per share) for the twelve months ended December 31, 2022.
Cash, cash equivalents and marketable securities totaled $13.5 million as of December 31, 2023.
About Oryzon
Founded in 2000 in Barcelona, Spain, Oryzon (ISIN Code: ES0167733015) is a clinical stage biopharmaceutical company and the European leader in epigenetics, with a strong focus on personalized medicine in CNS disorders and oncology. Oryzon’s team is composed of highly qualified professionals from the pharma industry located in Barcelona, Boston, and San Diego. Oryzon has an advanced clinical portfolio with two LSD1 inhibitors, vafidemstat in CNS and iadademstat in oncology, in several Phase II clinical trials. The company has other pipeline assets directed against other epigenetic targets like HDAC-6, where ORY-4001 has been nominated as clinical candidate for the treatment of certain neurological disorders such as CMT and ALS. In addition, Oryzon has a strong platform for biomarker identification and target validation for a variety of malignant and neurological diseases. For more information, visit www.oryzon.com
About Iadademstat
Iadademstat (ORY-1001) is a small oral molecule, which acts as a highly selective inhibitor of the epigenetic enzyme LSD1 and has a powerful differentiating effect in hematologic cancers (see Maes et al., Cancer Cell 2018 Mar 12; 33 (3): 495-511.e12.doi: 10.1016 / j.ccell.2018.02.002.). A FiM Phase I/IIa clinical trial with iadademstat in R/R AML patients demonstrated the safety and good tolerability of the drug and preliminary signs of antileukemic activity, including a CRi (see Salamero et al, J Clin Oncol, 2020, 38(36): 4260-4273. doi: 10.1200/JCO.19.03250). Iadademstat has shown encouraging safety and efficacy data in combination with azacitidine in a Phase IIa trial in elder 1L AML patients (ALICE trial) (see Salamero et al., ASH 2022 oral presentation). Iadademstat is currently being evaluated in combination with gilteritinib in the ongoing Phase Ib FRIDA trial in patients with relapsed/refractory AML with FLT3 mutations. Beyond hematological cancers, the inhibition of LSD1 has been proposed as a valid therapeutic approach in some solid tumors such as small cell lung cancer (SCLC), neuroendocrine tumors (NET), medulloblastoma and others. In a Phase IIa trial in combination with platinum/etoposide in second line ED-SCLC patients (CLEPSIDRA trial), preliminary activity and safety results have been reported (see Navarro et al., ESMO 2018 poster). Iadademstat is being evaluated in a collaborative Phase II basket study with the Fox Chase Cancer Center (FCCC) in combination with paclitaxel in R/R neuroendocrine carcinomas, and the company is preparing a new trial in combination with immune checkpoint inhibitors (ICI) in SCLC. Oryzon has entered into a Cooperative Research and Development Agreement (CRADA) with the U.S. National Cancer Institute (NCI) to collaborate on potential further clinical development of iadademstat in different types of solid and hematological cancers; a first trial in combination with ICI in SCLC is under preparation. In total iadademstat has been dosed so far to more than 130 cancer patients in four clinical trials. Iadademstat has orphan drug designation for SCLC in the US and for AML in the US and EU.
About Vafidemstat
Vafidemstat (ORY-2001) is an oral, CNS-optimized LSD1 inhibitor. The molecule acts on several levels: it reduces cognitive impairment, including memory loss and neuroinflammation, and at the same time has neuroprotective effects. In animal studies vafidemstat not only restores memory but reduces the exacerbated aggressiveness of SAMP8 mice, a model for accelerated aging and Alzheimer’s disease (AD), to normal levels and also reduces social avoidance and enhances sociability in murine models. In addition, vafidemstat exhibits fast, strong, and durable efficacy in several preclinical models of multiple sclerosis (MS). Oryzon has performed two Phase IIa clinical trials in aggressiveness in patients with different psychiatric disorders (REIMAGINE) and in aggressive/agitated patients with moderate or severe AD (REIMAGINE-AD), with positive clinical results reported in both. Additional finalized Phase IIa clinical trials with vafidemstat include the ETHERAL trial in patients with Mild to Moderate AD, where a significant reduction of the inflammatory biomarker YKL40 has been observed after 6 and 12 months of treatment, and the pilot, small-scale SATEEN trial in Relapse-Remitting and Secondary Progressive MS, where anti-inflammatory activity has also been observed. Vafidemstat has also been tested in a Phase II in severe Covid-19 patients (ESCAPE) assessing the capability of the drug to prevent ARDS, one of the most severe complications of the viral infection, where it showed significant anti-inflammatory effects in severe Covid-19 patients. Vafidemstat is being investigated in neuropsychiatric disorders in two double-blind, randomized, placebo-controlled Phase IIb trials: one in schizophrenia, named EVOLUTION (recruitment ongoing), and another one in Borderline Personality disorder (BPD), named PORTICO, recently finalized, with topline data and in the process of completing the full data analysis. Based on PORTICO’s topline results, the company is planning to request an End-of-Phase II meeting with the FDA to discuss options for a registrational Phase III trial in BPD. The company is also deploying a CNS precision medicine approach with vafidemstat in genetically-defined patient subpopulations of certain CNS disorders and is preparing a clinical trial in Kabuki Syndrome patients. The company is also exploring the clinical development of vafidemstat in other neurodevelopmental syndromes.
FORWARD-LOOKING STATEMENTS
This communication contains, or may contain, forward-looking information and statements about Oryzon, including financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, capital expenditures, synergies, products and services, and statements regarding future performance. Forward-looking statements are statements that are not historical facts and are generally identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although Oryzon believes that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Oryzon shares are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Oryzon that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the documents sent by Oryzon to the Spanish Comisión Nacional del Mercado de Valores (CNMV), which are accessible to the public. Forward-looking statements are not guarantees of future performance and have not been reviewed by the auditors of Oryzon. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date they were made. All subsequent oral or written forward-looking statements attributable to Oryzon or any of its members, directors, officers, employees or any persons acting on its behalf are expressly qualified in their entirety by the cautionary statement above. All forward-looking statements included herein are based on information available to Oryzon on the date hereof. Except as required by applicable law, Oryzon does not undertake any obligation to publicly update or revise any forward‐looking statements, whether as a result of new information, future events or otherwise. This press release is not an offer of securities for sale in the United States or any other jurisdiction. Oryzon’s securities may not be offered or sold in the United States absent registration or an exemption from registration. Any public offering of Oryzon’s securities to be made in the United States will be made by means of a prospectus that may be obtained from Oryzon or the selling security holder, as applicable, that will contain detailed information about Oryzon and management, as well as financial statements.