ORYZON First Half Ended June 30, 2020 Results and Corporate Update
MADRID, SPAIN and CAMBRIDGE, MA, UNITED STATES, July 22nd, 2020 – Oryzon Genomics, S.A. (ISIN Code: ES0167733015, ORY), a clinical-stage biopharmaceutical company leveraging epigenetics to develop therapies in diseases with strong unmet medical need, today reported financial results for the first half of 2020 and provided an update on recent developments.
Dr Carlos Buesa, Oryzon’s Chief Executive Officer, said, "The financial market confidence in Oryzon’s clinical programs has allowed us to raise new funds during this quarter in favorable conditions at a lower discount than the average observed in similar transactions. This extends our cash runway until Q1 2023, allowing the company to weather possible adverse market conditions due to the Covid-19 pandemic. The proceeds will support further advancement of our clinical programs after the exciting signals of clinical efficacy observed in both our iadademstat oncology and vafidemstat neurology programs, combined with good safety and tolerability profiles. We are especially excited with the initiation of our program in precision medicine in psychiatry with vafidemstat. Our collaboration with Hospital La Paz-INGEMM will be the first of a series of new partnerships in this area, which could help to pioneer a new approach to treating psychiatric disorders.”
Second Quarter and Recent Highlights
Iadademstat in oncology:
- Additional positive efficacy data from Phase II trial ALICE, investigating iadademstat in combination with azacitidine in acute myeloid leukemia (AML), was presented at the virtual EHA-2020:
- Robust signals of clinical efficacy, with reported objective responses in 10 out of 13 evaluable patients (77% ORR): of these, 60% were complete remissions (6CR/CRi).
- Rapid onset of clinical responses, with mean time to response (TTR) of 37 days. Longest remission was 488 days, still ongoing
- Several patients had also improved or overcome their dependency on blood transfusio
- Expect to present final data of the Phase II trial CLEPSIDRA, investigating iadademstat in small cell lung cancer (SCLC) in combination with standard-of-care platinum-etoposide, in September 2020 at the virtual ESMO conference
Vafidemstat in neurological disease:
- Entered pioneering precision medicine collaboration with the Institute of Medical and Molecular Genetics (INGEMM) of the La Paz University Hospital in Madrid in patients with Phelan-McDermid Syndrome (PMS). PMS is thought to be one of the causes of autism spectrum disorder. In this collaboration, a cognitive, behavioral and functional baseline assessment of PMS patients will be performed prior to a clinical study with vafidemstat in these patients.
- New project to explore the efficacy of vafidemstat in the treatment of schizophrenia announced in June, to receive € 0.7 million public funding from the Spanish Ministry of Science and Innovation. This project will be performed in collaboration with the Research Institute of Vall d’Hebrón (VHIR) in Barcelona and will explore the efficacy of vafidemstat in the treatment of patients with schizophrenia or related psychiatric disorders.
- Continuing the ETHERAL Phase IIa trial evaluating vafidemstat in mild and moderate AD patients, and the extension phase of the SATEEN Phase IIa clinical trial evaluating vafidemstat in MS in patients with the secondary progressive form of the disease up to a maximum of 18 months of vafidemstat treatment.
- Started a new study in severe Covid-19 patients, named ESCAPE. This is an open-label, randomized, double arm Phase II trial to assess the efficacy and tolerability of vafidemstat in combination with standard of care, to prevent progression to Acute Respiratory Distress Syndrome (ARDS). The study, which will enroll 20 patients per arm, is currently actively recruiting patients in several Spanish hospitals.
Financial Update: First Half 2020 Financial Results
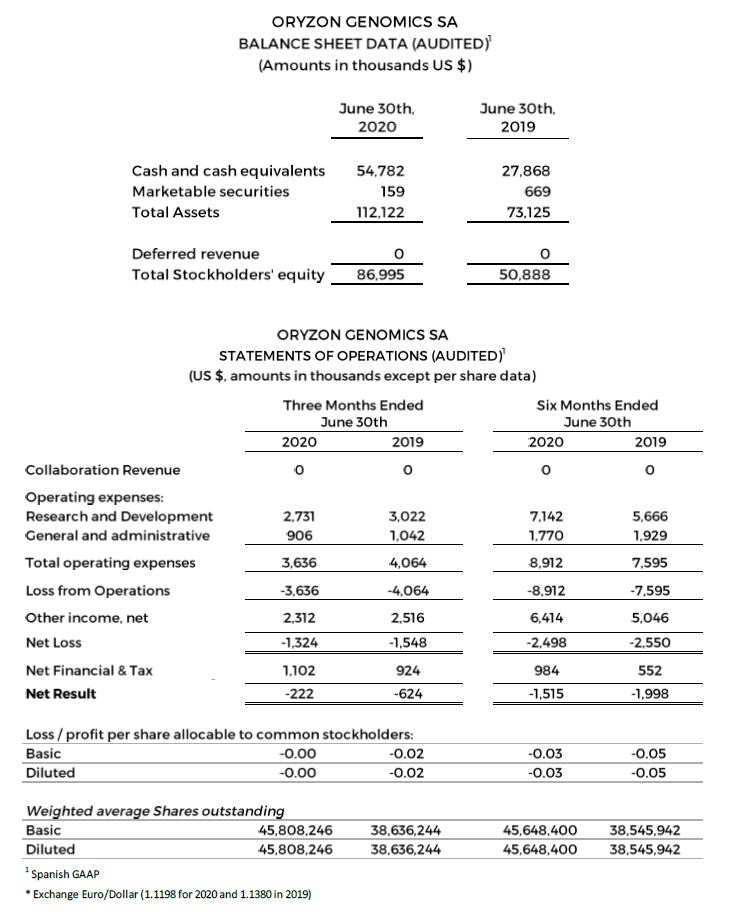
Research and development (R&D) expenses were $2.7 and $7.1 million for the first 3 and 6 months ended June 30, 2020 compared to $3.0 and $5.7 million for the last 3 and 6 months ended June 30, 2019. The $1.4 million increase was driven primarily by expenses associated with advancing the company’s clinical trials.
General and administrative expenses were $0.9 and $ 1.8 million for the first 3 and 6 months ended June 30, 2020 compared to the $1.0 and $1.9 million for the first 3 and 6 months ended June 2019.
Net losses were $1.3 and $2.5 million, respectively, for the first 3 and 6 months ended June 30, 2020 compared to net losses of $1.6 and $2.6 million for the first 3 and 6 months ended June 2019, representing increases of 14.5% and 2.0%, respectively.
Negative Net Result of $1.5 million (-$0.03 per share) for the 6 first months ended June 30, 2020, compared to a negative Net Result of $2.0 million for the first 6 months ended June 30, 2019.
Cash, cash equivalents and marketable securities totaled $54.9 million as of June 30, 2020, compared to $28.5 million as of June 30, 2019.
On June 25th, the company announced a Private Placement with international investors and issued 7,273,000 new common shares, at a price of EUR 2.75 per share, representing a 10.7% discount on the closing price of June 24. These generated gross proceeds of EUR 20 million (approximately $22.4 million at the exchange rate on that day).
About Oryzon
Founded in 2000 in Barcelona, Spain, Oryzon (ISIN Code: ES0167733015) is a clinical stage biopharmaceutical company considered as the European champion in Epigenetics. Oryzon has one of the strongest portfolios in the field. Oryzon’s LSD1 program has rendered two compounds, vafidemstat and iadademstat, in clinical trials. In addition, Oryzon has ongoing programs for developing inhibitors against other epigenetic targets. Oryzon has a strong technological platform for biomarker identification and performs biomarker and target validation for a variety of malignant and neurological diseases. Oryzon has offices in Spain and the United States. For more information, visit www.oryzon.com
FORWARD-LOOKING STATEMENTS
This communication contains, or may contain, forward-looking information and statements about Oryzon, including financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, capital expenditures, synergies, products and services, and statements regarding future performance. Forward-looking statements are statements that are not historical facts and are generally identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although Oryzon believes that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Oryzon shares are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Oryzon that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the documents sent by Oryzon to the Spanish Comisión Nacional del Mercado de Valores (CNMV), which are accessible to the public. Forward-looking statements are not guarantees of future performance and have not been reviewed by the auditors of Oryzon. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date they were made. All subsequent oral or written forward-looking statements attributable to Oryzon or any of its members, directors, officers, employees or any persons acting on its behalf are expressly qualified in their entirety by the cautionary statement above. All forward-looking statements included herein are based on information available to Oryzon on the date hereof. Except as required by applicable law, Oryzon does not undertake any obligation to publicly update or revise any forward‐looking statements, whether as a result of new information, future events or otherwise. This press release is not an offer of securities for sale in the United States or any other jurisdiction. Oryzon’s securities may not be offered or sold in the United States absent registration or an exemption from registration. Any public offering of Oryzon’s securities to be made in the United States will be made by means of a prospectus that may be obtained from Oryzon or the selling security holder, as applicable, that will contain detailed information about Oryzon and management, as well as financial statements.